House Finch Eye Disease: Outbreak, Then Understanding
By Rebecca Heisman
January 11, 2017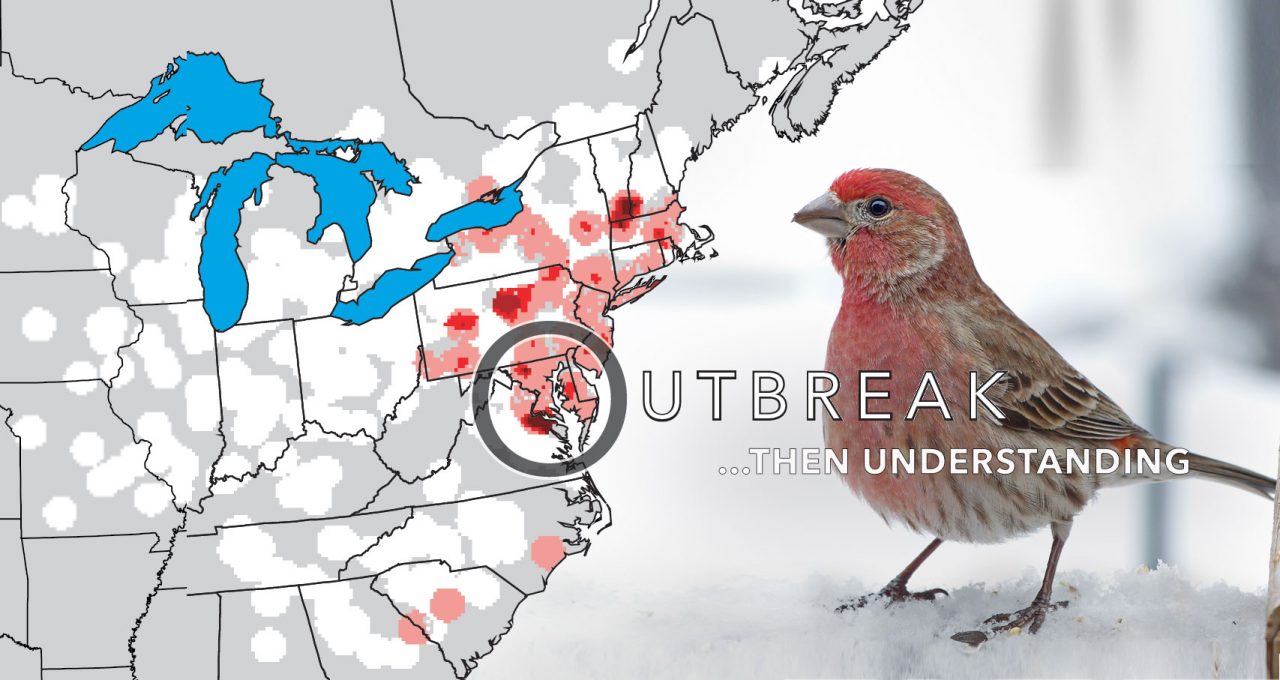
From the Winter 2017 issue of Living Bird magazine. Subscribe now.
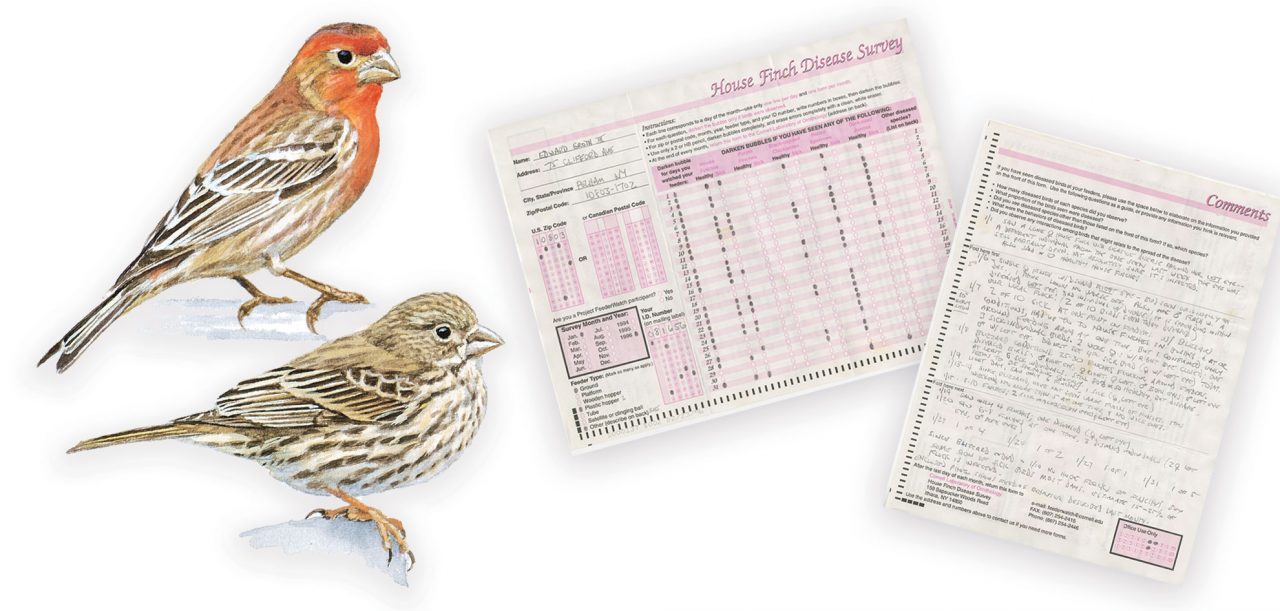
In February 1994, backyard bird watchers in Maryland noticed something strange. House Finches were showing up at feeders with eyes that were red and crusty, so swollen that they bulged out of the small birds’ heads.
In Ithaca, N.Y., volunteers at the Cornell Lab of Ornithology’s Project FeederWatch were sorting through data forms sent in by project participants, and they noticed a repeating theme among reports from the Baltimore/ Washington, D.C., area.
“It was the old handwritten report days, and we had a comments section for descriptions,” recalls former FeederWatch project manager Margaret Barker. “Our volunteers kept coming across mentions of House Finches with puffy eyes, some acting oddly at the feeders.”
After being alerted by the volunteers, Barker combed through the comments and found more sick finch reports— lots more, mostly from Maryland. But no one knew why the birds were getting sick, or what it meant.
Back in the early 1990s, the House Finch would have been a candidate for the title of most successful bird species in North America. Its populations were marching across the continent, and the little brown bird with the cheerful red head was a familiar sight at backyard bird feeders in the West and East.
Then a poultry pathogen made an unexpected cross-species leap, and House Finches debuted a new form of conjunctivitis. Ultimately, though, the outbreak for a beloved backyard bird species turned into the perfect opportunity for scientists to observe how disease spreads and evolves in a natural environment. Over the past two-and-a-half decades, Cornell University researchers and their colleagues have tracked the epidemic known as “House Finch eye disease” across the country. Their data are providing new insights that may be applicable to headline-making human epidemics such as Zika.
It all started with a Belgian ornithologist newly arrived in America and a mass-mailing of thousands of paper forms, a pre-Internet appeal for citizen scientists to collect the data that the professionals couldn’t.
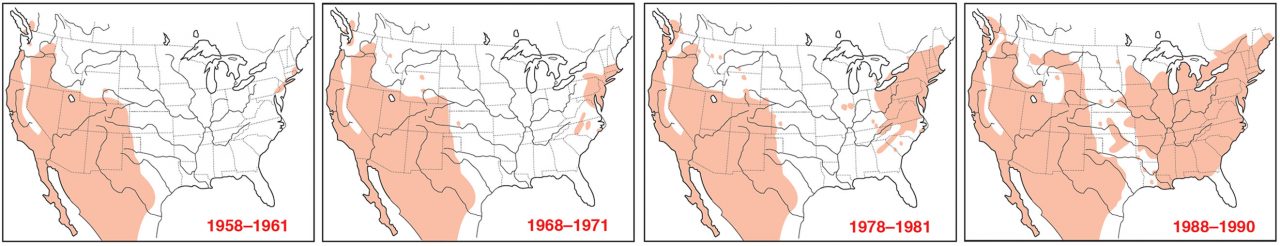
How House Finches Moved Across North America
House Finch are native to the deserts, grasslands, and chaparral of western North America. Around 1940, an illicit trade in pet “Hollywood finches” sprang up, with illegally trapped birds shipped east out of California. When a passing birder recognized the songbirds in a Brooklyn pet-shop window and reported them to the Audubon Society, federal wildlife officials moved to shut down the illegal trade. Soon pet-shop owners released their inventory in order to avoid prosecution, shooing the birds out the door and into the wilds of Long Island.
At first, this new population of House Finches remained small and contained—the harsh winter of 1947–48 left fewer than 50 of the desert-adapted birds clinging to survival in suburban New York City. But by the 1960s the eastern House Finch population was expanding rapidly, and by the mid-1990s they were taking over the continent—the introduced eastern population radiated out from New York, the native western population spread eastward, and the two populations met in the Midwest. Then along came the mysterious new eye disease epidemic.
Like House Finches, André Dhondt is not a native of the eastern U.S. In spring 1994, the Belgian ornithologist left a 20-year career at the University of Antwerp to come to the Cornell Lab of Ornithology. Dhondt was already a successful population ecologist when he arrived to take over as director of the Cornell Lab’s Bird Population Studies program, having run a decades-long experiment in his native country on competition between Great Tits and Blue Tits. He was looking for a new challenge, but he had no way of knowing that his career was about to become inextricably intertwined with the new disease alarming backyard bird watchers.
…by the mid-1990s they were taking over the continent. Then along came the mysterious new eye disease epidemic.
“As I arrived, there was a panic that this new disease, which at that time was unidentified, would spread to Neotropical migrants, they would take it back to South America, and everybody would die. I’m exaggerating,” he admits, “but there was general concern.”
With that concern came an unprecedented opportunity for scientists to track the spread of a new disease from almost the moment of its origin. Not only was House Finch eye disease easy to spot, eliminating the need to capture and test birds for diagnosis, but the Cornell Lab of Ornithology already had an army of thousands of backyard bird watchers across the U.S. and Canada submitting data to Project FeederWatch.
Within months of the initial reports of the disease’s emergence, Dhondt spent all of his $5,000 in start-up money for his new lab on printing 50,000 paper forms to send to FeederWatch participants, asking them for observations of House Finches. Then he waited.
“It was a perfect system,” says Dhondt. “Not for the birds, but for us.”
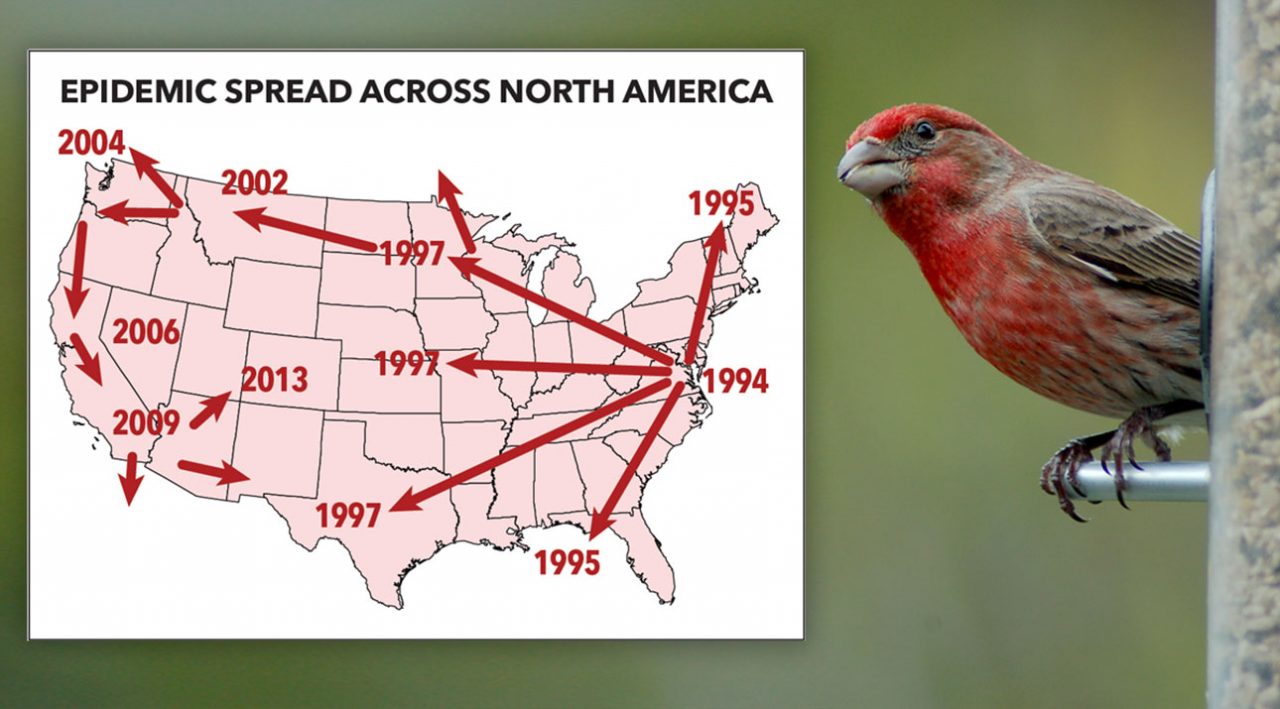
Dhondt’s Gamble Paid Off
Data for the newly christened House Finch Disease Survey soon poured in from citizen scientists across the country. Over the next 10 years, he amassed almost 300,000 monthly reports from 10,000 participants.
The culprit behind the new disease was identified from bacteria collected from infected birds’ lesions—Mycoplasma gallisepticum, a species of bacteria known to cause respiratory infections in chickens and turkeys. Genetic analysis suggested that M. gallisepticum jumped from poultry to wild songbirds unsuccessfully several times before a single strain of the bacteria finally adapted to persist in House Finches, possibly the result of some unlucky finch patient zero darting into a backyard flock of chickens to share their feed.
A year after it first emerged, the disease was reported in Ontario to the north, Virginia to the south, and Ohio to the west. In fall 1995, it was in Indiana, Michigan, and Illinois. By fall 1996, it had reached Wisconsin, Minnesota, and Iowa. In only two-and-a-half years, the conjunctivitis epidemic had spread throughout most of the eastern House Finch range.

Thanks to long-running volunteer bird surveys like the Christmas Bird Count, scientists had data on the host’s abundance both before and after the start of the epidemic—something almost unheard of in studies of wildlife diseases.
“In areas where there were a lot of birds around, it was very easy for the bacteria to hop from one House Finch to another. Infections were more rampant, and birds were dying at a higher rate,” explains Wesley Hochachka, Dhondt’s colleague at the Cornell Lab and partner on the House Finch eye disease research project. But after the initial period when the disease hit and populations declined, disease occurrence tailed off and populations stabilized at smaller sizes. Mycoplasma simply couldn’t jump from bird to bird at feeders as effectively when population densities were lower. It was one of the first large-scale examples of a disease regulating the population dynamics of its host.
The intersection of disease virulence and population dynamics opened up a host of new questions about bacterial mutations and pathogen evolution, but those were questions that citizen science alone couldn’t answer. And Dhondt and Hochachka—both population biologists by training—had never worked on disease research before House Finches. “My main experience with disease was being sick, up until the point that I came to Cornell,” says Hochachka.
So they formed a multidisciplinary team of scientists from several institutions, from a mathematical modeler at Princeton to a veterinary microbiologist at North Carolina State University, to tackle bigger inquiries into mycoplasma. The bigger team needed more money. Serendipitously, a major new funding source for disease research launched in 2000 when the National Science Foundation and the National Institutes of Health started a joint Ecology of Infectious Diseases program (later expanded to Ecology and Evolution of Infectious Diseases). The goal was to fill the gap between studies of disease in humans and in wildlife, funding research on natural systems that could enhance our understanding of both.
The team submitted a proposal to use House Finch eye disease as a system for exploring how diseases evolve and spread. They were funded in the very first round of the new grants. With the new influx of funding, the House Finch researchers expanded their fieldwork across North America to monitor changes in disease prevalence and finch abundance over time, accumulating 15,000 observations of 4,500 banded birds from intensive field studies in New York, Wisconsin, and Georgia. They also began experiments with birds in captivity to study the course of the disease in controlled conditions, observing how it passed from bird to bird, whether birds recovered, and how they fared if infected for a second time.
The new research program showed that wild birds could recover from the disease, and studies on captive birds confirmed that birds retained partial immunity for at least a year once they’d pulled through. Crucially, the House Finch research team built a mathematical model to generalize what they learned and apply it to other disease systems. Ultimately, people who can’t tell a finch from a robin may reap the benefits of the House Finch studies, because it’s giving science new insights into diseases that affect a species we all care about—humans.

A Microscopic Arms Race
According to the world health organization, human infectious diseases are spreading faster and emerging more quickly than ever before, with new diseases identified at the rate of one per year since the 1970s. In just the last five years, more than 1,000 epidemics have affected humans around the world.
In 2002—almost exactly 10 years after House Finch eye disease first appeared in Maryland—a smaller epidemic was detected among backyard birds in Missoula, Montana. M. gallisepticum was now in the western range of the House Finch, probably introduced by sick finches from the East that were infected with a relatively mild strain and were still healthy enough to fly from town to town. The disease’s spread offered new insights into how pathogens evolve over time.
“Like Alice in Wonderland, we’ve got a ‘Red Queen’ situation here where the bacteria are running as fast as they can to keep up.”
~Wesley Hochachka
In both the East and the West, M. gallisepticum’s virulence increased after emergence, likely spurred by the immunity that House Finches develop once exposed. Because finches’ immune systems became better and better at dealing with the disease, M. gallisepticum had to become more virulent over time.
“One of the parts of the story that’s recently become apparent is that we’ve got an arms race happening, where the finches are able to develop immunity to the bacteria and it seems like the only way that the bacteria can then work around the immunity is to evolve to cause more severe disease,” explains Hochachka. “Like Alice in Wonderland, we’ve got a ‘Red Queen’ situation here where the bacteria are running as fast as they can to keep up.”
The birds, he says, are essentially able to vaccinate themselves through their own immune response, but because the pathogen is able to evolve rapidly in return, the prevalence of the disease is remaining relatively stable over time.
“The human implication, if we were to extrapolate, is that if you’re vaccinating a human population against some sort of a pathogen and you don’t do it completely enough to eradicate it, you may end up with enough little living laboratories among the hosts for the bacteria to live and evolve,” Hochachka says. “What might pop out in the end is a more virulent pathogen, able to cause more severe disease.”
“The current outbreak of Zika is due to some kind of a mutation that occurred somewhere in Asia that suddenly made Zika much more virulent, and now it’s spreading very rapidly,” says Samuel Scheiner, the NSF program officer who manages the Cornell Lab’s grant for the finch research. “The idea is that you can use things like the House Finch project as a way to study those processes, and then you can apply that knowledge to things like the Zika outbreak.”
This interdisciplinary research project is helping to train new generations of disease ecologists who are comfortable blurring the boundaries between population biology, epidemiology, and veterinary science. Dhondt’s House Finch research is currently on its third round of funding from the Ecology and Evolution of Infectious Diseases program, and scientists who began their careers as graduate students in Dhondt’s laboratory have now moved on to head disease ecology labs of their own at institutions such as Virginia Tech.
As for Dhondt, even after 20 years he still isn’t out of questions for more House Finch experiments. “The next step is to see to what extent other pathogens in the system play a role,” he says, nodding to the idea that other pathogens could partner up with M. gallisepticum to double-team a finch’s immune system. “That’s why we’ve started looking at avian malaria.”
And what would he have thought, on his way to America two decades ago, if he’d known that finches with crusty eyes were going to change the course of his career? “I would have thought it was really cool,” he says. “I came to Cornell when I was 50, a midlife crisis kind of thing, and I had to decide what I would do here. I was going to do something new. I didn’t know exactly what, but this happened.
“And one thing you learn in life is, if you have a chance to do something challenging, do it.”
Rebecca Heisman is a freelance science writer and the communications assistant for ornithology journals The Auk and The Condor. She lives in Walla Walla, Washington.

All About Birds
is a free resource
Available for everyone,
funded by donors like you
American Kestrel by Blair Dudeck / Macaulay Library